Plan for Pennsylvania: Testing Strategy
Testing to identify COVID-19 is a critical component to our mitigation strategies. There are varied approaches to COVID-19 testing that include differences among test type, criteria for testing and measures for success. Diagnostic testing determines if an individual has an active infection with COVID-19, while serologic (antibody) testing determines if an individual was previously infected at some point. The commonwealth's vision is to ensure that anyone experiencing symptoms of COVID-19 is able to receive a diagnostic test.
What is the Testing Strategy?
The enhanced testing strategy focuses on three pillars: ensuring testing is accessible for all Pennsylvanians with symptoms of COVID-19; available by increasing supply and building community capacity and adaptable to the evolving landscape of the virus and data.
Testing Accessible for All Pennsylvanians
It is a top priority to ensure that all people in Pennsylvania who are experiencing symptoms of COVID-19 or who have been exposed to someone with COVID-19 have access to diagnostic testing. To ensure a healthy Pennsylvania for all, especially during these unprecedented times, the Wolf Administration will only partner with organizations and entities throughout our response to COVID-19 that have an established commitment to non-discriminatory practices. In addition to continuing robust testing at the Department of Health's State Laboratory, the commonwealth has partnered and collaborated with existing community resources, such as retail pharmacies, Pennsylvania's Community Health Centers or Federally Qualified Health Centers (FQHCs), to ensure that testing is widely available.
The number of cases in communities will continue to be closely monitored to ensure that testing sites are situated appropriately in areas with the greatest need. For example, if there is a substantial outbreak in a long-term care facility, testing resources will be targeted to that facility as needed. The department will listen to community partners, county/municipal health departments and local health care leaders to guide a localized strategy.
In Pennsylvania, all major health insurance providers, including Medical Assistance contractors, cover medically appropriate laboratory testing for COVID-19. Additionally, the Pennsylvania Department of Health continues to perform testing free of charge.
Increasing Testing Availability
An increase in laboratory equipment, test kits and specimen collection sites is critical to enhancing testing capacity across the state. In 2020, the Department of Health closely collaborated with the Department of Community and Economic Development, other state agencies and federal partners to seek additional testing resources. A group of interdisciplinary professionals from various state agencies were brought together to specifically focus on ensuring an accessible, available, and adaptable testing throughout Pennsylvania.
Using the Department of Health's incident command structure, the Department of Health had the responsibility of determining who should be tested and what tests are needed; the Department of Community and Economic Development was working proactively to seek out FDA-approved testing resources while working closely with Pennsylvania-based private sector partners to develop new testing technology; and the Pennsylvania Emergency Management Agency assisted with getting tests into the hands of communities. This team also identified necessary funding to meet the needs of this accessible, available and adaptable testing strategy. As more testing sites become available, they will be added to the Commonwealth's
map of testing sites. Pennsylvania is currently tracking 973 testing sites across the state.
Ensuring the Plan is Adaptable
The Department recognizes that the COVID-19 pandemic will continue to evolve rapidly as the tools, research and data are improved to protect public health. The Administration is deeply committed to a data-driven, community-based approach to addressing COVID-19. This means we must remain flexible in our strategy. As more information becomes available, we will continue to refine our approach based on scientific evidence and input from communities in Pennsylvania. The Commonwealth has and will continue to quickly adapt to changes in the testing environment. Further, the Commonwealth will continue to review where the greatest need for testing is and leverage mobile testing capabilities when possible to ensure flexibility in the testing response.
What Progress has Been Made Related to Diagnostic Testing?
Testing capacity throughout the commonwealth continues to improve. The Department of Health's State Laboratory testing capacity has increased dramatically from being able to conduct up to 4 diagnostic tests per day in February 2020 to approximately 1,500 tests per day in 2021. As of March 2023, the state laboratory has tested over 506,032 specimens in total for COVID-19. Testing capacity has also greatly expanded among commercial laboratories, in addition to the state laboratory. On average, there was 8,820 RT-PCR tests reported per day for the month of March.
Antigen tests have also been added to help detect COVID-19. A majority of the antigen tests that have been granted an Emergency Use Authorization (EUA) by the FDA are rapid point of care tests. While the Department of Health's State Laboratory is not conducting antigen tests, other laboratories across the state are using these tests. It should be noted that while antigen tests are a quicker, less expensive alternative to RT-PCR tests, the "gold standard" for diagnosing a person with COVID-19 is still the RT-PCR test. There were 43,624 antigen tests reported for the month of February. For additional information regarding antigen tests, please see the CDC's Interim Guidance for Rapid Antigen Testing for SARS-CoV-2.
The following graphs outline the number of COVID-19 diagnostic tests conducted daily throughout Pennsylvania by both commercial and state laboratories for the month of February and the number of COVID-19 diagnostic tests conducted monthly throughout Pennsylvania by both commercial and state laboratories. This data includes RT-PCR tests and antigen tests.
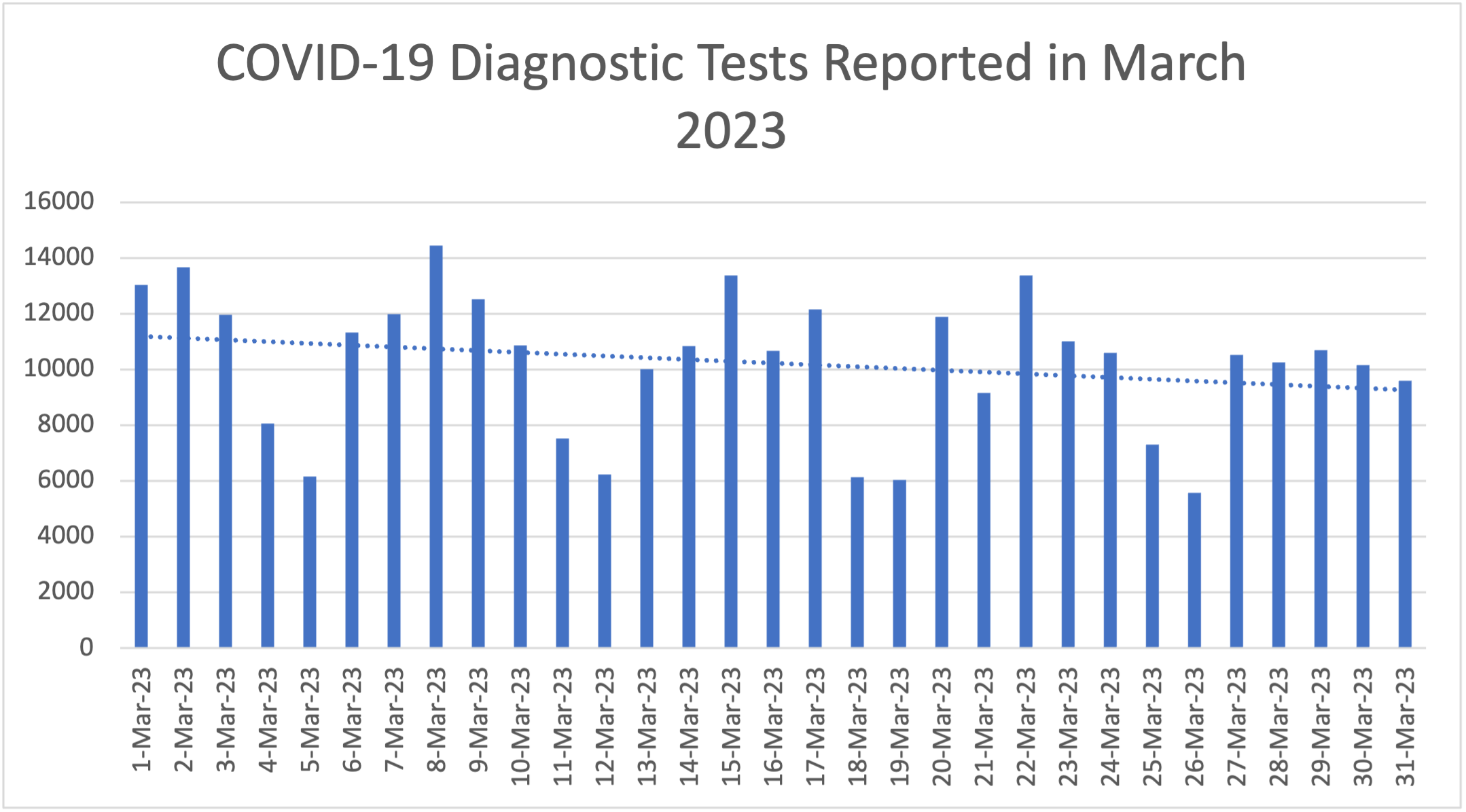
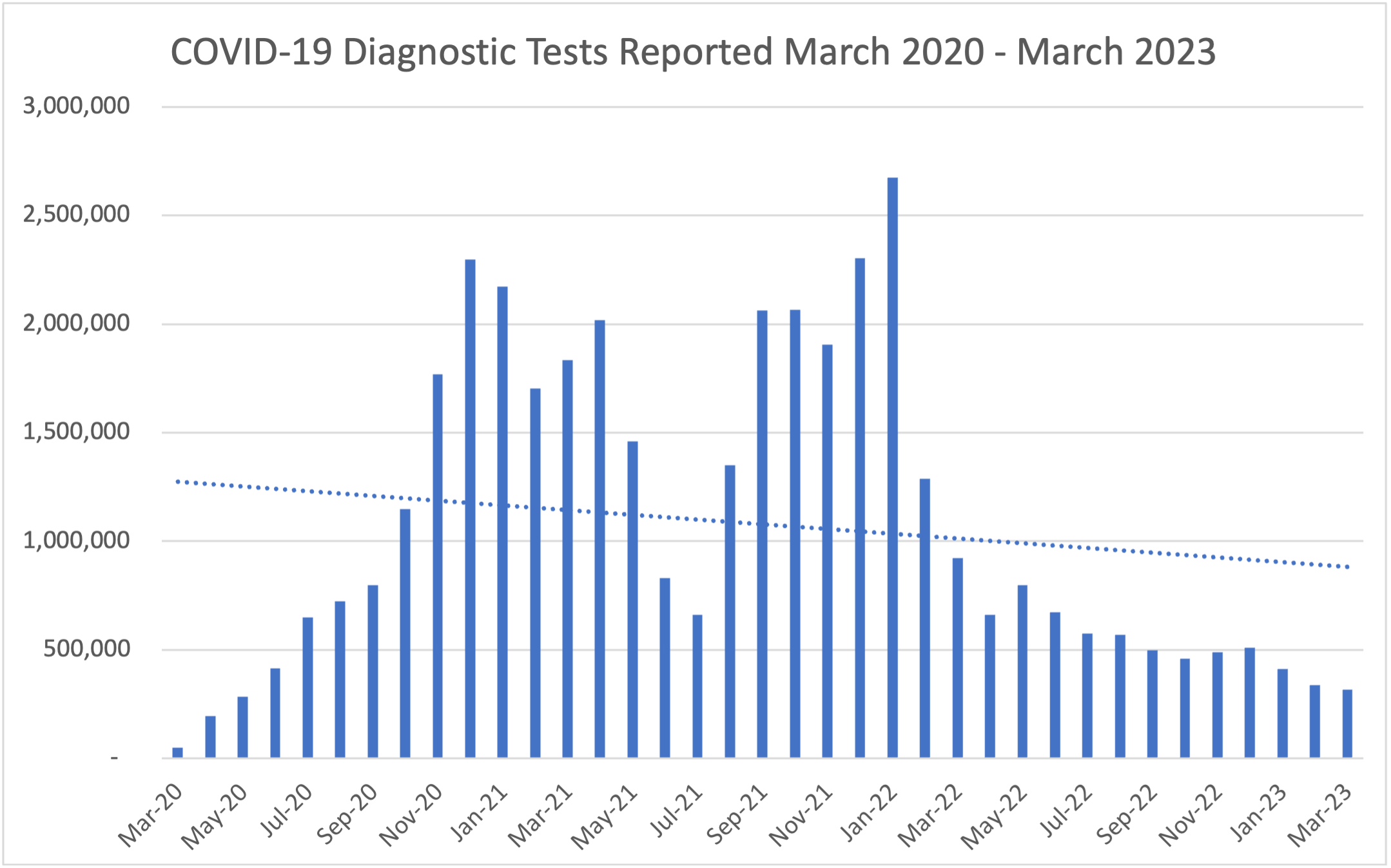
Through this enhanced strategy, the Governor set a goal to have a testing site within 45 miles of 90 percent of people in Pennsylvania, and for an equivalent of 2% of the population in every region throughout the commonwealth to be tested per month. This goal was achieved by coordinating with private industry, the PA National Guard, county and municipal health departments as well as hospital systems and Federally Qualified Heath Centers (FQHCs) to provide testing at over 400 testing sites. In addition, the Department teamed up with the PA National Guard and CVS Pharmacies to provide testing at long term care facilities.
With the vaccine administration, the demand for testing has decreased, but testing is still extremely important. The below chart outlines the test rate per 100,000 people for the month of March, how many tests were completed in the month of March, and how many tests were completed throughout this pandemic. These numbers include RT-PCR tests and antigen tests.
| NW | 882,499 | 2,560 | 22,594 | 2,556,164
|
|---|
| SW | 2,641,545 | 1,915 | 50,593 | 7,820,099
|
|---|
| NC | 697,154 | 3,216 | 22,422 | 2,371,414
|
|---|
| SC | 1,727,120 | 2,801 | 48,374 | 5,130,582
|
|---|
| NE | 1,610,439 | 2,564 | 41,295 | 4,833,626
|
|---|
| SE | 5,243,232 | 2,513 | 131,771 | 17,184,190
|
|---|
| Total | 12,801,989 | 2,477 | 317,049 | 38,896,075 |
|---|
DOH is continuing to review available research on tests that identify previous exposure to COVID-19. Due to limited data on how much protection antibodies provide against reinfection, serology testing is not being implemented on a wide scale throughout the commonwealth. However, this testing strategy will be adapted as more information and resources become available.
While DOH is not implementing serology testing, these tests are being administered and reported to the Department of Health. Throughout the pandemic, 602,381 serology tests have been reported to the Department.
Resources for More Information
Coronavirus Symptoms & Testing:
https://www.health.pa.gov/topics/disease/coronavirus/Pages/Symptoms-Testing.aspx
Testing for COVID-19:
https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html
COVID-19 Serology Surveillance Strategy: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/serology-surveillance/index.html