NOTE: As a result of the Federal data requirement, the Acting Secretary of the Pennsylvania Department of Health has
rescinded the March 16, 2021
Order that required skilled nursing care facilities to complete the COVID-19 vaccine needs assessment survey.
COVID-19 Vaccination Resources
COVID-19 Vaccine for Staff and Residents of Long-Term Care Facilities
NOTE: This strategy is for long-term care facilities and long-term care pharmacies in all counties in Pennsylvania except Philadelphia. The Philadelphia Department of Public Health operates their own Immunization Information System (PhilaVax). More information about Philadelphia's vaccine programs can be found at
https://vax.phila.gov/index.php/covid-19/.
The Department of Health will be leveraging federal COVID-19 vaccine allocations through the Federal Pharmacy Partnership (FPP) for Long-Term Care Program to ensure the provision of vaccine to staff and residents of long-term care facilities on an ongoing basis. The long-term care facilities impacted by this strategy includes those licensed by either the Department of Health or the Department of Human Services. The Centers for Disease Control and Prevention (CDC) runs the FPP.
This approach allows long-term care facilities to request COVID-19 vaccine from eligible Pennsylvania long-term care pharmacies who in turn order from Federal Long-Term Care Pharmacy Partners (Partners) receiving federal vaccine allocations. The Partners consist of three Group Purchasing Organizations (GPOs) and CVS/Omnicare. The three GPOs are GeriMed, Innovatix, and Managed Health Care Associates, Inc. (MHA). Participation of the long-term care pharmacies already in their network is dependent upon them becoming eligible pharmacies through the FPP
and enrolling in Pennsylvania Immunization Electronic Registry System (PIERS). To ensure access to all long-term care pharmacies for this vaccine strategy, the three GPOs are willing to add more long-term care pharmacies into their network.
Contact information for the Partners is available on CDC's website. Each Partner has its own process for enrollment, and CDC allows eligible long-term care pharmacies to be added on the 1st and 15th of each month.
Each long-term care pharmacy will be required to sign CDC's
COVID-19 Vaccination Program Provider Agreement for Pharmacies Serving Long-Term Care Facilities (available through the Partner) to participate and be deemed eligible. Eligible long-term care pharmacies must also be enrolled in PIERS. An eligible long-term care pharmacy will then administer the vaccine on-site at the long-term care facility or may use contractors (including a long-term care facility) to perform some or all of the eligible pharmacy's duties under the Provider Agreement. The eligible long-term care pharmacy must ensure that any contractor performs its duties in full compliance with the Provider Agreement.
While the first phase of the FPP in Pennsylvania (executed by CVS and Walgreens) limited the participation to long-term care facilities that met specific criteria, there is no such criteria for this phase. If an eligible long-term care pharmacy wants to serve a facility, they may.
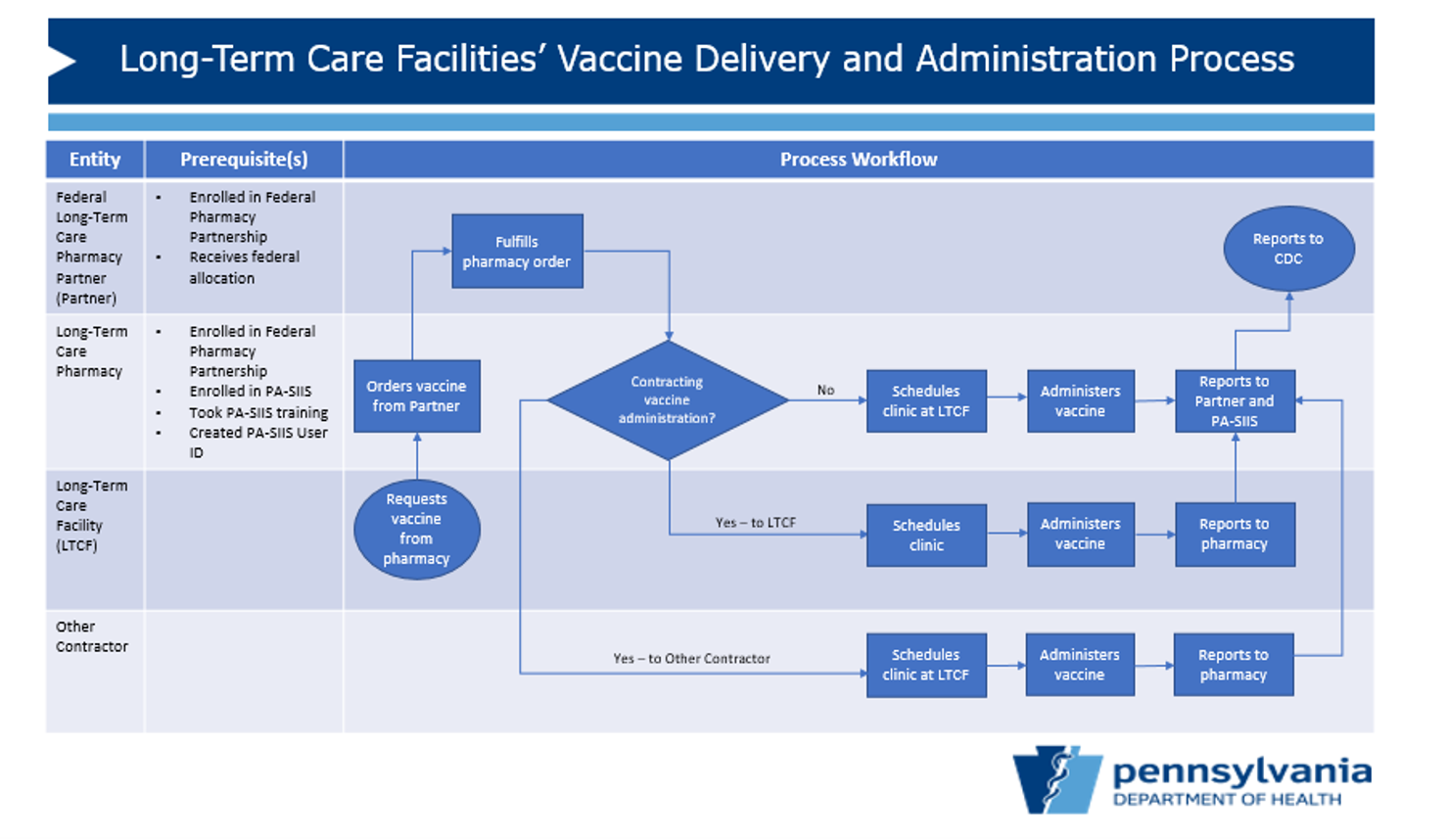
A high-level overview of the process is shown below.
Checklists have been developed that may be helpful to long-term care facilities and long-term care pharmacies in navigating this process. Links to resources and forms are embedded in the checklists to provide additional information. The checklists may be found at the links below:
Questions may be directed via email to
ra-dhcovidvax@pa.gov.